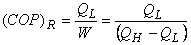Tuesday, 20 May 2025
Tuesday, 13 August 2024
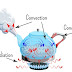
Heat Transfer – Conduction, Convection and Radiation
Heat Transfer – Conduction, Convection and Radiation
Thermal Energy Transfer
- Thermal energy can be transferred in
three main ways: conduction, convection, and radiation.
Heat Conduction
- Conduction is the movement of heat within
a solid object.
- Conduction occurs when objects are
touching, such as a kettle on a stove.
- Heat from the flame moves through the
metal of the kettle to the water inside.
- Butter melting on a frying pan is another
example of heat conduction.
Heat Conduction
- Heat conduction occurs through direct
contact when heat moves from a warmer object to a cooler object.
- When you lick an ice cream, it feels cold
because heat conducts from your tongue to the ice cream.
- Heat conduction is the movement of heat
in liquids and gases.
Convection
- Convection is the movement of heat in
liquids and gases.
- In a hot air balloon, the air is heated
by the burner and rises inside the balloon.
- As the hot air rises, the cooler air
falls, creating a current within the balloon.
- The convection current causes thermal
energy in the air to spread. Cooling a Room with an Air Conditioner
- On a hot day, an air conditioner can be
used to cool down a room
- The cold air that blows out from the air
conditioner circulates the room and creates convection currents
Radiation
- Radiation is the process of heat moving from a warmer object to a cooler object without affecting the medium in between.
- Radiation uses electromagnetic waves to emit heat waves that can be reflected, absorbed, or transmitted through a colder object.
- This process doesn't require a medium, unlike conduction and convection.
- For example:- The sun's rays can warm the Earth even after passing through the mesosphere.
Heat Transfer through Conduction and
Convection
- Heat moves from the warmer air to the
colder air, which makes the air in the room cooler
- For the thermal energy to move by
conduction and convection, it must travel through matter
Heat Transfer between the Sun and the
Earth
- The heat transfer between the sun and the
earth occurs through conduction and convection heat Transfer and the Sun's
Warmth
- The sun warms the Earth and other planets
through radiation.
- Radiation is the transfer of energy
through electromagnetic waves.
Radiation vs. Convection
- Radiation is the direct transfer of heat,
as seen when a fireplace warms a person.
- Convection is the transfer of heat
through the movement of a medium, such as the air being warmed in a room by a
fireplace.
- Both radiation and convection play a role
in how the sun's heat reaches and warms the Earth.
Radiant Heat
- Radiant heat is the warmth felt when
placing your hands near an electric heater or other heat source.
- Radiant heat is the direct transfer of
thermal energy through electromagnetic waves, without needing a physical
medium.
- This is the primary mechanism by which
the sun's heat reaches and warms the Earth and other planets in the solar
system. Heat Transfer
- Heat can be transferred in three main
ways: conduction, convection, and radiation.
- Conduction is the transfer of heat
through direct contact between objects or materials, such as heat moving
through the metal of a kettle to the water inside.
- Convection is the transfer of heat
through the movement of a fluid, such as the circulation of hot water within a
kettle.
- Radiation is the transfer of heat through
electromagnetic waves, such as the heat from a heater or campfire.
Heat Transfer in a Kettle
- When water in a kettle is heated, thermal
energy moves through the metal of the kettle by conduction.
- The thermal energy then moves around the
water through convection, as the water circulates and mixes.
- Finally, the heat leaves the kettle and
is transferred to the surrounding environment through radiation.
Conclusion
- Heat can be transferred through various
mechanisms, including conduction, convection, and radiation.
- These processes can be observed in
everyday examples, such as water heating in a kettle.
Wednesday, 2 January 2019
Limitations of the first law
Limitations of the first law
- It doesn't tell us the direction in which heat flows when they are in contact.
- It doesn't tell about the final temperature of two bodies when they are in direct contact.
- It doesn't tell about the entropy of the system.
Sunday, 7 October 2018
Refrigerator
 and
and represents the amount of energy absorbed as heat from the low-temperature reservoir and the energy rejected as heat to the high-temperature reservoir respectively, Let W be the work done on the device to accomplish the task.
represents the amount of energy absorbed as heat from the low-temperature reservoir and the energy rejected as heat to the high-temperature reservoir respectively, Let W be the work done on the device to accomplish the task.
(1)
|
(2)
|
(3)
|
 since
since  (heat) transferred to the system cannot be completely converted to work in a cycle. Therefore is
(heat) transferred to the system cannot be completely converted to work in a cycle. Therefore is less than unity. A heat engine can never be 100 efficient. Therefore
less than unity. A heat engine can never be 100 efficient. Therefore ., there has always to be a heat rejection. Thus a heat engine has to exchange heat with two reservoirs, the source and the sink. This experience leads to the proposition of the second law of thermodynamics which has been stated in several different ways.
., there has always to be a heat rejection. Thus a heat engine has to exchange heat with two reservoirs, the source and the sink. This experience leads to the proposition of the second law of thermodynamics which has been stated in several different ways.Monday, 24 September 2018
Intensive and Extensive properties with examples
PROPERTIES OF SYSTEMS
- Mass: This gives the idea of how much of the initial matter was contained in the system and how much is left after the process is complete.
- Volume: This gives an idea of the matter's dimension and what will be the final dimension after the process is over.
- Internal energy: It is the total energy contained in to create the thermodynamic system but excludes the energy to displace the system’s surroundings. It has two major components of kinetic energy and potential energy due to the movement of particles and the static electric power of the atoms contained in them.
- Heat contents: Under a given pressure, the heat content or Enthalpy is a measure of the total energy of a thermodynamic system. It includes internal energy which is required to create a system and establish its volume and pressure.
- Free energy: It is the energy in the physical system which can be converted into work.
- Entropy: It is a thermodynamic property that is used to determine the energy available for useful work in a thermodynamic process.
- Heat capacity: Heat capacity or thermal capacity is the measurable physical quantity that gives an idea of the amount of heat required to change a substance’s temperature by a given range.
- Density: The density of a material is defined as the ratio between its volume and the matter contained in or mass.
- Molar property: Molar property mainly consists of the detailing of molar volume, molar energy, molar entropy, and molar heat capacity, and all these are quantified from the point of moles of the substance involved.
- Surface tension: It is a property of a liquid surface that helps in resisting any kind of external force applied to it.
- Viscosity: It is a measurable internal quantity of a fluid that resists its flow.
- Specific heat: It is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
- Thermal conductivity: Thermal conductivity (λ) is the intrinsic property of a material that relates to its ability to conduct heat.
- Refractive index: The measure of the speed of light in a medium is referred to as the refractive index of that medium.
- Pressure: It is the perpendicular force acting per unit area on the surface of an object.
- Temperature: It is the property of the matter which quantitatively expresses the coldness or hotness of a substance.
- Boiling point: It is the temperature of the substance at which the vapor pressure of the liquid equals environmental pressure.
- Freezing point: It is the temperature at which a liquid composition solidifies under a given pressure.
- Vapour pressure of a liquid: It is defined as the equilibrium pressure above its liquid resulting due to the evaporation of liquid
Monday, 17 September 2018
Heat | गर्मी
Heat
Friday, 31 August 2018
Thermal reservoir
Thermal reservoir
 |
| Thermal reservoir |
Popular Posts
-
Thermal reservoir A thermal energy reservoir (TER) is defined as a large body of infinite heat capacity, which is capable of absorb...
-
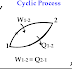
Cyclic process :- If a system performs the number of processes so that it comes back again to the initial state then the system the number...
-
All-in-One Online Calculator Toolbox 📏 Length Converter mm cm m km inch ft mile m...
-
Heat Transfer – Conduction, Convection and Radiation Thermal Energy Transfer - Thermal energy can be transferred in three main ways: c...
-
Zeroth Law :- When two bodies or systems are in thermal equilibrium with a third one then they are in thermal equilibrium with each other....
-
Limitations of the first law The 1st law of thermodynamics is the law of conservation of energy . It doesn't specify the direction ...
-
PROPERTIES OF SYSTEMS A property of a system is a characteristic of the system which depends upon its state, but not upon how the stat...
-
Refrigerator A refrigerator is a cyclically operating device which absorbs energy as heat from a low-temperature body and rejects ener...
-
Heat Heat - Heat is energy. Sun is the natural source of heat energy Definition:- Heat is the form of energy which produces the sensa...
-
Thermodynamics Thermodynamics :- Definition 1:- Thermodynamics is the branch of physical science that deals with the relations...
Blogger templates
Search This Blog
Translate
Popular Posts
-
Thermal reservoir A thermal energy reservoir (TER) is defined as a large body of infinite heat capacity, which is capable of absorb...
-
Cyclic process :- If a system performs the number of processes so that it comes back again to the initial state then the system the number...
-
All-in-One Online Calculator Toolbox 📏 Length Converter mm cm m km inch ft mile m...