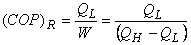PROPERTIES OF SYSTEMS
A property of a system is a characteristic of the system which depends upon its state, but
not upon how the state is reached. There are two sorts of property :
1. Intensive properties. These properties do not depend on the mass of the system.
Examples: Temperature and pressure.
OR
An extensive system property depends upon the total amount of material in the system. Mass, volume, internal energy, heat contents, free energy, entropy, and heat capacity are all extensive properties.
- Mass: This gives the idea of how much of the initial matter was contained in the system and how much is left after the process is complete.
- Volume: This gives an idea of the matter's dimension and what will be the final dimension after the process is over.
- Internal energy: It is the total energy contained in to create the thermodynamic system but excludes the energy to displace the system’s surroundings. It has two major components of kinetic energy and potential energy due to the movement of particles and the static electric power of the atoms contained in them.
- Heat contents: Under a given pressure, the heat content or Enthalpy is a measure of the total energy of a thermodynamic system. It includes internal energy which is required to create a system and establish its volume and pressure.
- Free energy: It is the energy in the physical system which can be converted into work.
- Entropy: It is a thermodynamic property that is used to determine the energy available for useful work in a thermodynamic process.
- Heat capacity: Heat capacity or thermal capacity is the measurable physical quantity that gives an idea of the amount of heat required to change a substance’s temperature by a given range.
2. Extensive properties. These properties depend on the mass of the system. Example :
Volume. Extensive properties are often divided by the mass associated with them to obtain the intensive
properties. For example, if the volume of a system of mass m is V, then the specific volume of
matter within the system is V
m = v which is an intensive property.
OR
An intensive property is defined as a property that is independent of the amount of material in the system. Density, molar property, surface tension, viscosity, specific heat, thermal conductivity, refractive index, pressure, temperature, boiling point, freezing point, and vapor pressure of a liquid are all intensive properties.
- Density: The density of a material is defined as the ratio between its volume and the matter contained in or mass.
- Molar property: Molar property mainly consists of the detailing of molar volume, molar energy, molar entropy, and molar heat capacity, and all these are quantified from the point of moles of the substance involved.
- Surface tension: It is a property of a liquid surface that helps in resisting any kind of external force applied to it.
- Viscosity: It is a measurable internal quantity of a fluid that resists its flow.
- Specific heat: It is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
- Thermal conductivity: Thermal conductivity (λ) is the intrinsic property of a material that relates to its ability to conduct heat.
- Refractive index: The measure of the speed of light in a medium is referred to as the refractive index of that medium.
- Pressure: It is the perpendicular force acting per unit area on the surface of an object.
- Temperature: It is the property of the matter which quantitatively expresses the coldness or hotness of a substance.
- Boiling point: It is the temperature of the substance at which the vapor pressure of the liquid equals environmental pressure.
- Freezing point: It is the temperature at which a liquid composition solidifies under a given pressure.
- Vapour pressure of a liquid: It is defined as the equilibrium pressure above its liquid resulting due to the evaporation of liquid
3. Specific property: An extensive property expressed per unit mass of the system
Example- specific energy, specific entropy
4: Molar property: The ratio of extensive property to mole number is known as the molar property
A thermodynamic system depending upon interactions between system and surroundings can be classified as an open system, closed system, or isolated system.
 and
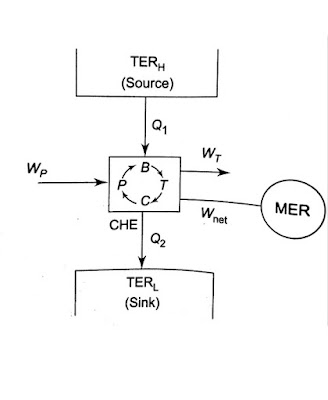
and represents the amount of energy absorbed as heat from the low-temperature reservoir and the energy rejected as heat to the high-temperature reservoir respectively, Let W be the work done on the device to accomplish the task.
represents the amount of energy absorbed as heat from the low-temperature reservoir and the energy rejected as heat to the high-temperature reservoir respectively, Let W be the work done on the device to accomplish the task.
 since
since  (heat) transferred to the system cannot be completely converted to work in a cycle. Therefore is
(heat) transferred to the system cannot be completely converted to work in a cycle. Therefore is less than unity. A heat engine can never be 100 efficient. Therefore
less than unity. A heat engine can never be 100 efficient. Therefore ., there has always to be a heat rejection. Thus a heat engine has to exchange heat with two reservoirs, the source and the sink. This experience leads to the proposition of the second law of thermodynamics which has been stated in several different ways.
., there has always to be a heat rejection. Thus a heat engine has to exchange heat with two reservoirs, the source and the sink. This experience leads to the proposition of the second law of thermodynamics which has been stated in several different ways.